SARS-CoV-2 Spike protein (RBD, His & Avi tag)
| ¥3800 | |
| Z03483 | |
|
|
|
|
|
|
|
|
|
| ¥3800 | |
| Z03483 | |
|
|
|
|
|
|
|
|
|
| Species | SARS-CoV-2 | ||||||
| Protein Construction |
|
||||||
| Purity | > 95% as analyzed by SDS-PAGE | ||||||
| Endotoxin Level | < 0.2 EU/μg of protein by gel clotting method | ||||||
| Biological Activity | SARS-CoV-2 Spike protein (RBD, His & Avi tag) can bind with Human ACE2 in functional ELISA assay. | ||||||
| Expression System | 293 Cells | ||||||
| Theoretical Molecular Weight | 30 kDa | ||||||
| Formulation | Supplied as a solution in PBS pH 7.2. | ||||||
| Concentration | Please refer to the COA for the specific lot. | ||||||
| Storage & Stability | Upon receiving, this product remains stable for up to 6 months at -20°C or below. Avoid repeated freeze-thaw cycles. |

»

»
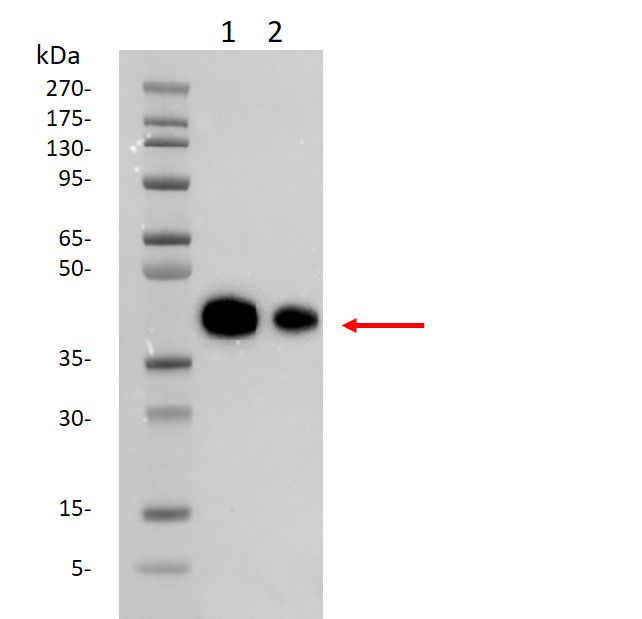
Western blot analysis of SARS-CoV-2 Spike protein (RBD, His & Avi tag)
(GenScript, Z03483) using S1 Antibody(GenScript, A02053).
Lane 1: 10 ng of Z03483
Lane 2: 5 ng of Z03483
»
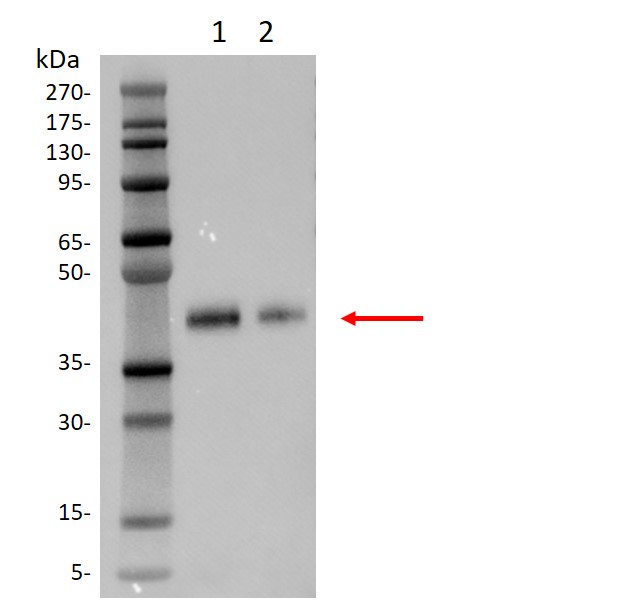
Western blot analysis of SARS-CoV-2 Spike protein (RBD, His & Avi tag)
(GenScript, Z03483) using His Antibody(GenScript, A00186).
Lane 1: 10 ng of Z03483
Lane 2: 5 ng of Z03483
»
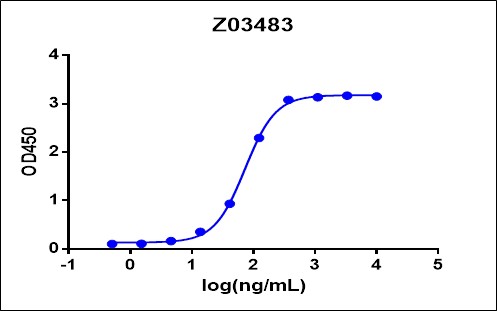
Immobilized ACE-2 Fc Chimera, Human (Cat. No. Z03484) at 2 μg/mL can bind SARS-CoV-2 Spike protein (RBD, His & Avi Tag) (Cat. No. Z03483) with a serial dilution.
THETM His Tag Antibody [HRP], mAb, Mouse (Cat.No.A00612) is used as a secondary antibody (0.1 μg/mL).
»
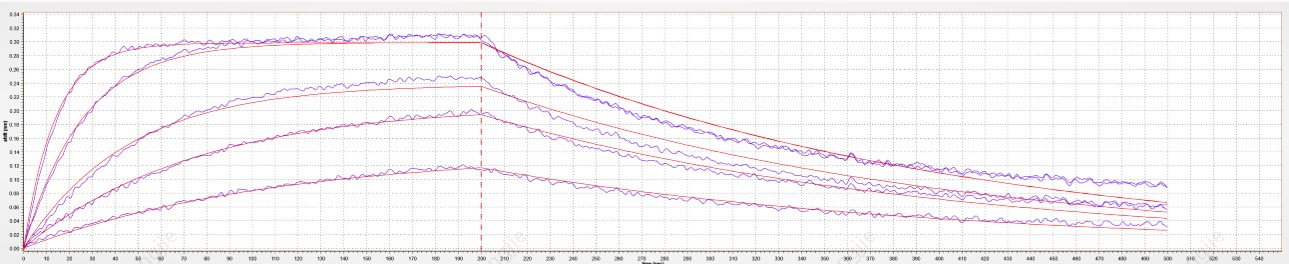
Loaded ACE-2 Fc Chimera, Human (CHO-expressed) Protein (Cat. No. Z03516) on Protein A Biosensor, can bind SARS-CoV-2 Spike protein (RBD, His & Avi Tag) (Cat. No. Z03483) with an affinity constant of 12.5 nM as determined in BLI assay. »
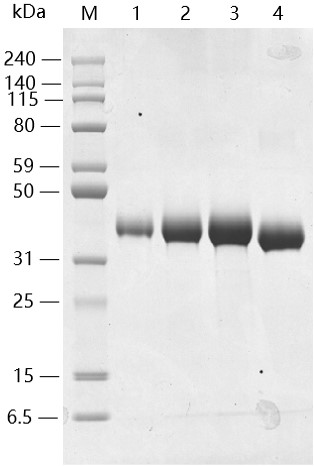
Lane 1: 1μg of SARS-CoV-2 Spike protein (RBD, His & Avi tag), reducing(R)
Lane 2: 3μg of SARS-CoV-2 Spike protein (RBD, His & Avi tag), reducing(R)
Lane 3: 5μg of SARS-CoV-2 Spike protein (RBD, His & Avi tag), reducing(R)
Lane 4: 5μg of SARS-CoV-2 Spike protein (RBD, His & Avi tag), non-reducing(NR)
> 95% as analyzed by SDS-PAGE
»
| Target Background | SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) also known as 2019-nCoV (2019 Novel Coronavirus) is a virus that causes illnesses ranging from the common cold to severe diseases. SARS-CoV-2 Spike Protein is composed of S1 domain and S2 domain. S1 contains a receptor-binding domain (RBD) that can specifically bind to angiotensin-converting enzyme 2 (ACE2), the receptor on target cells. It is believed that SARS-CoV-2 Spike Protein (RBD) has potential value for the diagnosis of the virus. |
| Synonyms | SARS-CoV-2 SP RBD; 2019-nCoV SP RBD |
For laboratory research use only. Direct human use, including taking orally and injection and clinical use are forbidden.