| Product Description |
Bevacizumab,
with the brand name Avastin, is a humanized monoclonal antibody of IgG1
isotype. It targets the vascular endothelial growth factor A (VEGF-A), preventing
its activation of VEGF receptor and inhibiting malignant cell growth and blood
vessel formation. Bevacizumab is approved for the treatment of patients with
cervical cancer, metastatic colorectal cancer, primary peritoneal cancer and so
on.
GenScript has developed and validated the
Bevacizumab Pharmacokinetic ELISA Kit for quantitative measurement of Bevacizumab
in cynomolgus monkey serum and plasma, following the ICH
M10 and the FDA bioanalytical method validation guidance for industry. Its
precision, accuracy, dilutional linearity, specificity, selectivity, stability,
and hook effect were acceptable according to the guidances [1-4]. The Bevacizumab ELISA kit is a validated tool for quantifying
both Bevacizumab and its biosimilars in biological matrices, facilitating drug
research and development. |
| LLOQ |
39.06 ng/mL
|
| ULOQ |
2500 ng/mL
|
| Precision |
Intra-assay: CV≤10%
Inter-assay: CV≤15%
|
| Minimum required dilution (MRD) |
1:50 selected by cynomolgus monkey plasma
|
| Specificity |
No cross-reactivity at 25,000 ng/mL of Human IgG1
|
| Hook Effect |
Not observed at 200,000 ng/mL of Bevacizumab
|
| Kit Contents |
| Component |
Quantity/Size |
Part No. |
| Capture Plate |
1 plate |
M1-80 |
| Standard Stock |
1 vial (50 μL) |
M1-10 |
| Sample Dilution Buffer |
1 bottle (60 mL) |
M1-60 |
| Biotin Anti-Bevacizumab Antibody |
1 bottle (12 mL) |
M1-20 |
| Streptavidin-HRP |
1 bottle (12 mL) |
M1-30 |
| 20× Wash Solution |
1 bottle (60 mL) |
M1-70 |
| TMB Solution |
1 bottle (12 mL) |
A1-40 |
| Stop Solution |
1 bottle (6 mL) |
A1-50 |
| Plate Sealer |
2 pieces |
N/A |
|
| Storage |
The
unopened kit is stable for at least 12 months from the date of manufacture at
2°C to 8°C, and the opened kit is stable for up to 1 month from the date of
opening at 2°C to 8°C. |

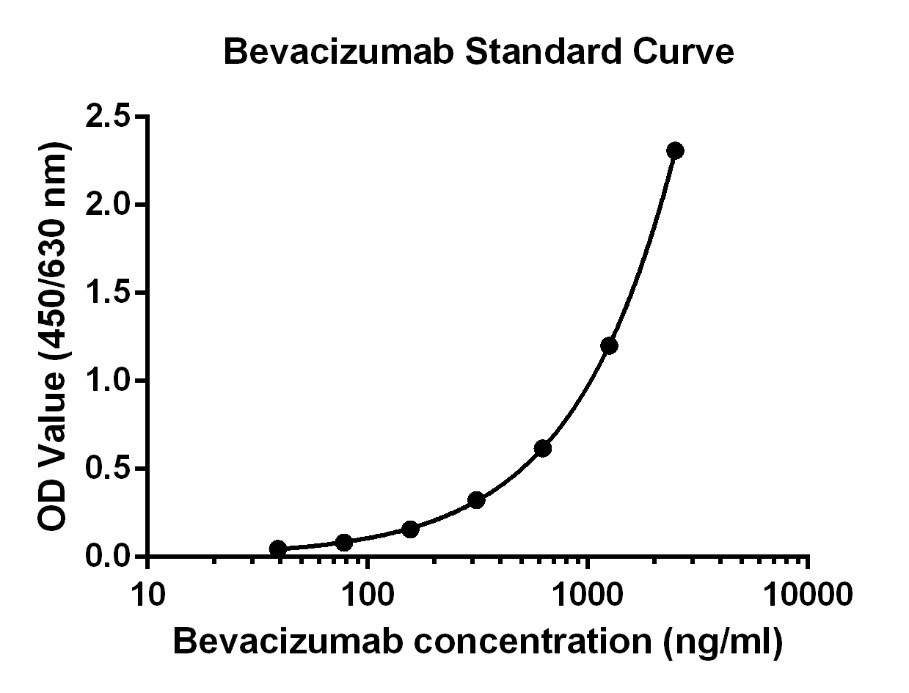
Figure 1: Bevacizumab ELISA kit standard curve.
A set of Bevacizumab calibration standards from 39.06 ng/mL to 2500 ng/mL was then diluted with Sample Dilution Buffer with a volume ratio of 1:50.
For research use only.
Not intended for human and animal therapeutic or diagnostic use.